Background: AML and MDS cells accumulate mutations over years or even decades. In the current WHO 2022 classification, some mutations are listed as “defining genetic abnormalities” (WHO DGA). A long phase of clonal hematopoiesis may precede some, but not all, AML or MDS. There are multiple scenarios in which order clonal hematopoiesis, WHO DGAs, and other mutations can occur. We analyzed whole-genome sequencing data from 583 patients with AML or MDS subtypes defined by a mutation and elucidated their role in the hierarchical sequence of mutations.
Aim: (1) We compared patients with AML or MDS arising from clonal hematopoiesis to those with a founder mutation in a WHO DGA gene (e.g. NPM1). (2) We analyzed which other mutations may occur before WHO DGAs.
Methods and patients: We analyzed 583 patients with AML or MDS who were classified into a WHO 2022 DGA class: MDS with low blasts and SF3B1 mutation (MDS- SF3B1, n=160), MDS with biallelic TP53 inactivation (MDS-bi TP53, n=40), AML, myelodysplasia-related (AML-MR with focus on mutations, n=151), AML with NPM1 (n=171), and AML with CEBPA (n=61). Amplification-free WGS was performed (median coverage > 100x). Genes with known relevance to myeloid malignancies were analyzed (n=47). We assumed that the mutation with the highest variant allele frequency (VAF) evolved first in the malignant process (+/- 5%). VAF was corrected for copy number changes (including the presence of one X chromosome in males) and copy-neutral loss of heterozygosity (CN-LOH). The following variant callers were used: Strelka2 (mutations and VAF), Pindel (for FLT3-ITD), HadoopCNV (CN-LOH) and GATK4 (CNV).
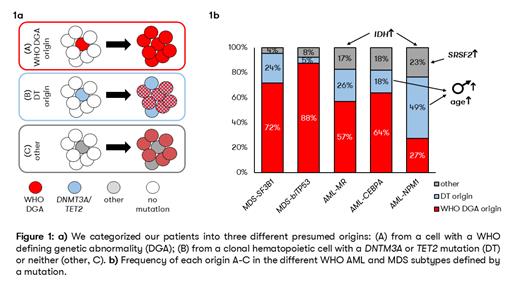
Results: We classified patients into three groups (Figure 1a): (A) 322/583 (55%) of malignancies originate from a cell with a WHO DGA; (B) 174/583 (30%) of malignancies originate from a clonal hematopoietic cell with a DNTM3A or TET2 mutation (DT origin); (C) the remaining 87/583 (15%) of AML and MDS originate from a cell with a different initial mutation (“other”). The frequency of patients with the highest VAF in a WHO DGA varied between subtypes: MDS-bi TP53: 88%, MDS- SF3B1: 72%, AML- CEPBA: 64%, AML-MR: 57% and AML- NPM1: 27%. The highest number of patients with a disease putatively arising from DNMT3A or TET2 mutated clonal hematopoiesis was found in AML- NPM1 (49%) and the lowest in MDS-bi TP53 (5%) (for details, see Figure 1b). The group of patients with the highest VAF in neither WHO DGA nor DNMT3A/ TET2 (“other”) accounted for 17-23% of AML and only 4% and 8% of MDS- SF3B1 andMDS-bi TP53 respectively. IDH1 or IDH2 had the highest VAF in 17/583 patients (3%), especially AML- NPM1 (n=8) and AML-MR (n=7). Mutations in the spliceosome genes had the highest VAF in 12/583 patients (2%), with the combination of SRSF2 in AML- NPM1 in particular (n=6).
In the following, we compared DT origin and WHO DGA origin. The differences did not affect all AML and MDS subtypes to the same extent. Patients with AML- CEBPA and DT origin were a median of 19 years older than patients with an initial CEBPA mutation (75 vs. 56 years). In AML- NPM1, the difference in median age was 11 years (71 vs. 60 years). In both groups, patients with DT origin are also predominantly male (73% vs. 44% for AML- CEBPA and 54% vs. 45% for AML- NPM1). Age and sex differences were not observed in AML-MR, MDS- SF3B1 and MDS-bi TP53 with or without DT origin. Overall survival (OS) was shorter in AML patients with DT origin compared to WHO DGA (0.7 vs. 1.8 years; p=0.073), but not in MDS (7.4 vs. 6.1 years; p=0.669). However, in a multivariate Cox analysis, including DT origin and age, age remained the only significant factor for OS.
Conclusion: Using VAF to model the hierarchy of mutation evolution shows that for the majority of patients, WHO-defining mutations are early events, supporting their role as defining genetic abnormalities and (potential) drivers. However, other initial mutations occur in DNMT3A and TET2, indicating AML or MDS arising from clonal hematopoiesis,but also IDH1/ IDH2 or spliceosome genes. How these diseases will be classified in the future should be actively discussed, and aspects such as targeted therapies (with IDH inhibitors) or a second hematologic neoplasm after clonal hematopoiesis should be considered.
Disclosures
Baer:MLL Munich Leukemia Laboratory: Current Employment. Huber:MLL Munich Leukemia Laboratory: Current Employment. Hoermann:MLL Munich Leukemia Laboratory: Current Employment. Meggendorfer:MLL Munich Leukemia Laboratory: Current Employment. Hutter:MLL Munich Leukemia Laboratory: Current Employment. Kern:MLL Munich Leukemia Laboratory: Current Employment, Other: Equity Ownership. Haferlach:MLL Munich Leukemia Laboratory: Current Employment, Other: Equity Ownership. Haferlach:MLL Munich Leukemia Laboratory: Current Employment, Other: Equity Ownership.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal